-
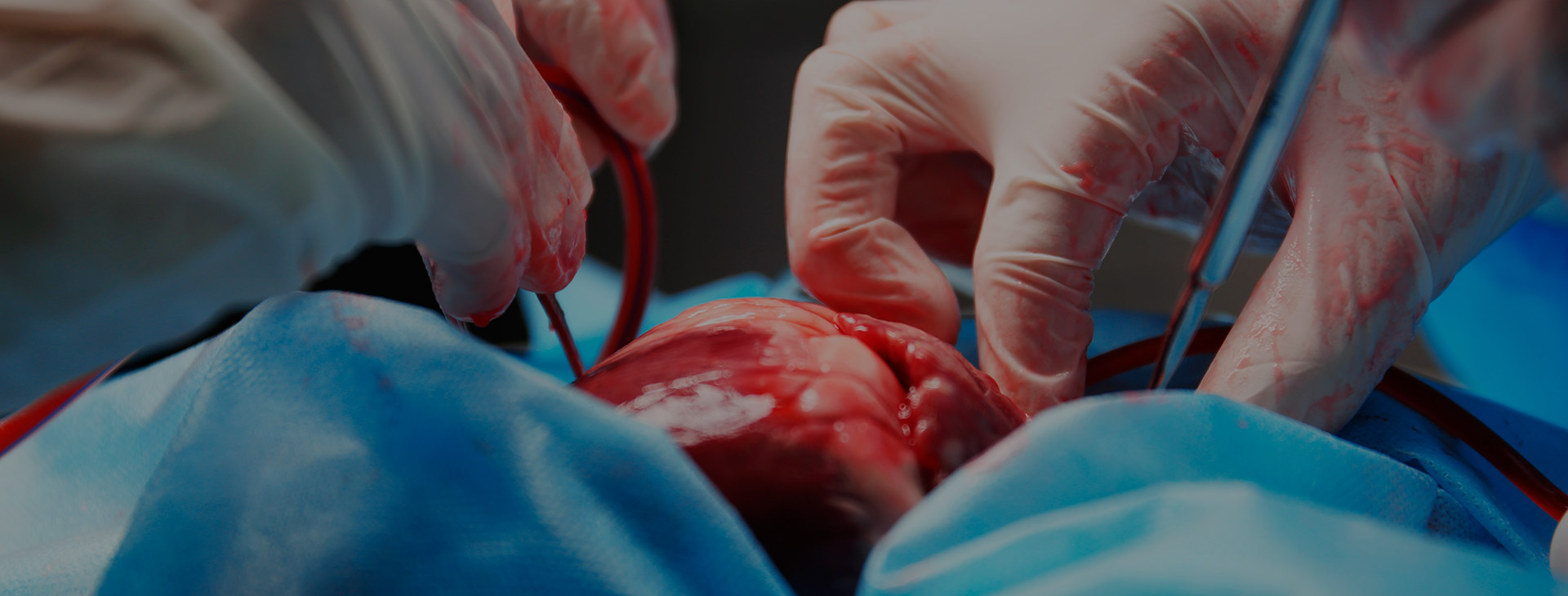
- The law firms of Flaherty McCarthy LLP and Waddell Phillips P.C. have commenced a multi-million dollar proposed Class Proceeding against LIVANOVA PLC, SORIN GROUP DEUTSCHLAND GMBH and SORIN GROUP USA, INC. seeking damages arising from the negligent design, testing, marketing and sale of the defendants’ Sorin 3T System. This is a medical device regulated in Canada pursuant to the Medical Devices Regulation, SOR/98-282, which is a Regulation to the Food and Drugs Act, RSC 1985, c F 27.The Sorin 3T System is used during cardiac surgery to regulate blood temperature by circulating water through tubes into a heat exchanger where blood is pumped into separate chambers during surgery. As the water passes through the water tanks and other areas it is aerosolized into a vapor. The Plaintiff alleges that this vapor containing NTM which exits from the device and is pushed into the ambient air of the operating room through the System’s exhaust fan. When placed in an operating room, the contaminated vapor from the System directly enters the sterile surgical field and the patient’s open body through their open surgical site.The primary bacteria at issue, inter alia, M. Chimaera and M. Abscessus, are subspecies of Nontuberculous Mycobacterium (“NTM”) that occurs naturally in the environment and rarely causes illness. NTM poses a unique risk to patients whose organs and chest cavities are directly exposed to the bacteria during surgery. Because NTM is a slow growing bacterium, it generally takes anywhere from two weeks to five years before manifestation of an NTM infection, which most commonly results in pulmonary or cardiovascular disease. Symptoms of NTM infection are frequently non specific and may include any of the following: fever, pain, heat or pus around a surgical incision, wound healing issues, night sweats, joint and muscle pain, weight loss, and fatigue. The diagnosis of an NTM infection requires targeted culturing and/or molecular diagnostic testing. While an NTM infection diagnosed early on may be successfully treated with a series of antibiotics, there is a significant risk of death in cases with delayed diagnoses and/or in individuals with considerably weakened immune systems.
- A copy of the Fourth Amended Statement of Claim can be found here. The allegations contained in the Statement of Claim have not yet been proven in Court.
- An application for funding for this action was heard by the Class Proceedings Fund on April 18, 2018. The funding application was successful. The Class Proceedings Fund will provide some funding for the costs of disbursements incurred in prosecuting the case, and will provide an indemnity to the plaintiffs for any adverse cost awards made by the court. In exchange, the Fund will be entitled to receive 10% of the net proceeds of any judgment or settlement achieved for the class.
- We have prepared a PowerPoint Presentation which provides further important information to Class Members which can be found here.
- This matter was certified as a class proceeding by the Ontario Superior Court of Justice on May 21, 2021. The ORDER and REASONS FOR DECISION can be accessed here.
- The Court approved this LONG-FORM NOTICE in ENGLISH and FRENCH advising you of the certification and the right of any class member to opt-out of the class proceeding.
- Class Counsel has also released this PRESS RELEASE to provide further notice.
- CBC’s The National aired a report on January 19, 2022 regarding the Livanova (Sorin) 3T Heater-Cooler Unit.
- As a class member, you do not have to do anything more to be involved in this lawsuit. You will be a member of the class unless you choose to opt-out in accordance with the Order of the Court.
- If you have been diagnosed with an M Chimaera infection, or are concerned that you may have been infected, then please contact us so that we can add you to the list of interested and affected class members to ensure that you will get direct notice of significant steps in the proceeding as the case progresses.